Raman spectroscopy is a non-invasive chemical analysis method. It is a vibrational spectroscopy like infrared (IR) spectroscopy which provides a simultaneous characterization of the chemical composition of a material, its environment or its degree of oxidation.
LISE has a high-level instrumental platform for chemical analysis at a nanoscale.
Principe
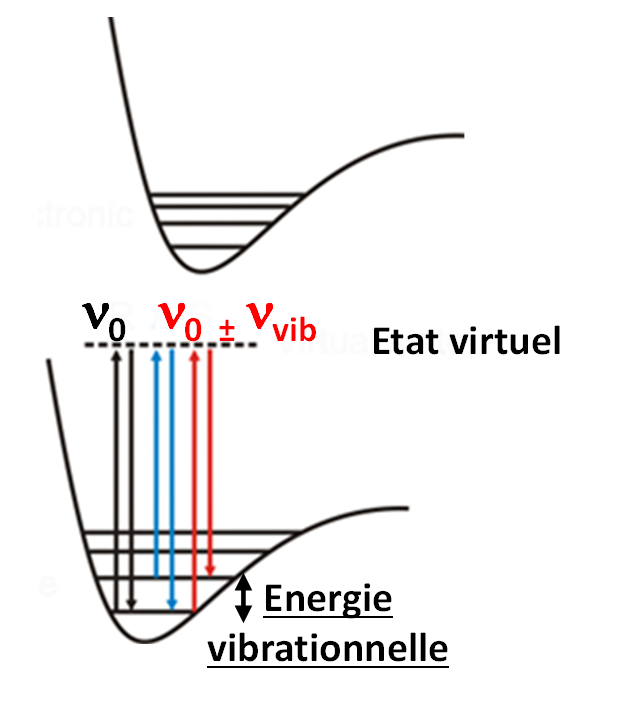 Raman spectroscopy is a scattering and not an absorption spectroscopy unlike IR. Raman photons are emitted when a sample is illuminated by a laser source (UV-visible-IR) through an inelastic scattering of light. The gain or loss of energy of inelastic photons emitted in relation to incident photons is translated on Raman spectra by a frequency shift. The Raman bands observed at a given frequency shift (ν0 - νinelast) correspond to the energy difference between the vibrational levels of the molecule analysed.
Raman spectroscopy is a scattering and not an absorption spectroscopy unlike IR. Raman photons are emitted when a sample is illuminated by a laser source (UV-visible-IR) through an inelastic scattering of light. The gain or loss of energy of inelastic photons emitted in relation to incident photons is translated on Raman spectra by a frequency shift. The Raman bands observed at a given frequency shift (ν0 - νinelast) correspond to the energy difference between the vibrational levels of the molecule analysed.
Avantages
- Raman vibrational signatures are therefore complementary to those observed in IR but have the advantage of not being impacted by the presence of water (no absorption and weak water diffusion signal when illuminated by visible light). Raman spectroscopy is therefore suitable for in situ analysis and electrochemical measurements.
- Due to stricter selection rules than in IR (variation in polarisability and not dipole moment), transitions are less frequent and chemical signatures are all the more clear.
- Its association with confocal microscopy also makes it possible to establish a chemical mapping with a much better lateral resolution than in IR, around 300 nm with traditional apparatus (limitation by diffraction).
Limitations
- The phenomenon of Raman scattering is very inefficient: one Raman photon for 107 incident photons. The effective diffusion cross-section of the studied material is therefore crucial in Raman microscopy and it may be difficult to obtain a spectroscopic signature for 1D and 2D materials other than carbon nanotubes and graphene sheets.
- The autofluorescence of the samples can dominate or even completely overwrite the Raman signal. The use of a near infrared exciter (785 nm) limits this problem.
- The signal obtained in Raman spectroscopy is extremely sensitive to the polarization of light, the wavelength used and the quality of the optical signal acquisition chain (filters, CCD, etc.).
Enhanced Raman Spectroscopy (SERS)
The low sensitivity of Raman spectroscopy has been compensated by the introduction of surface enhanced Raman spectroscopy (SERS). This methodology is based on the use of substrates with nanostructures or decorated with gold or silver nanoparticles. The free electrons of the metal oscillate in these Localized Surface Plasmon Resonance (LSPR) nanostructures, resulting in a strong localized amplification of the Raman signal of nearby compounds up to 1012, allowing the detection of single molecules.
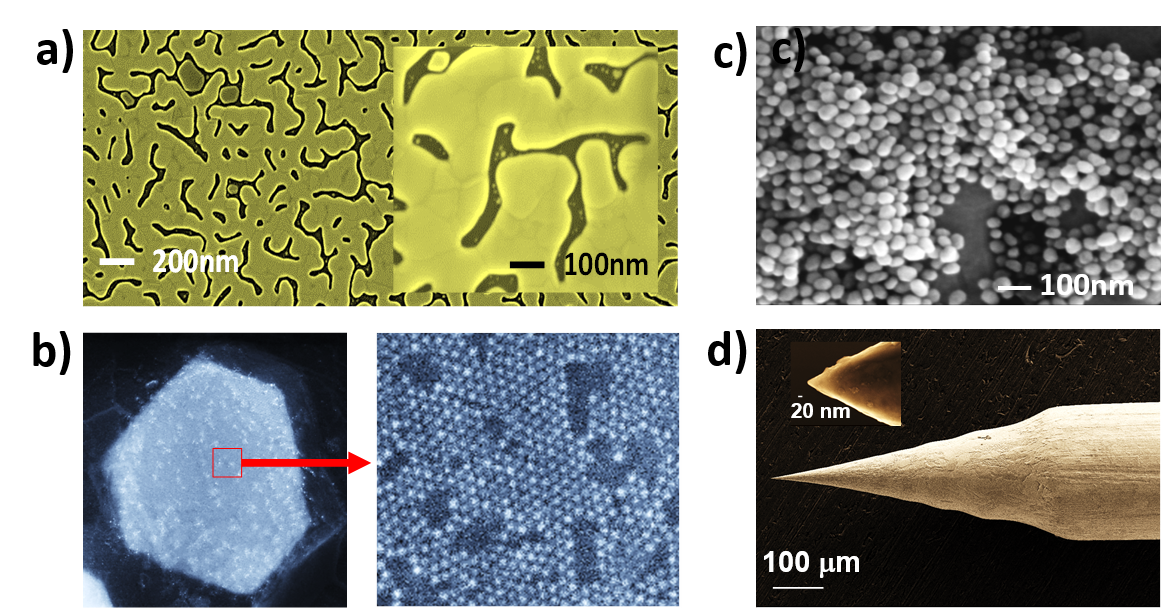
Coupled with local probe microscopy and/or electrochemistry, Raman spectroscopy becomes a powerful tool for structure analysis and reactivity at the nanoscale.