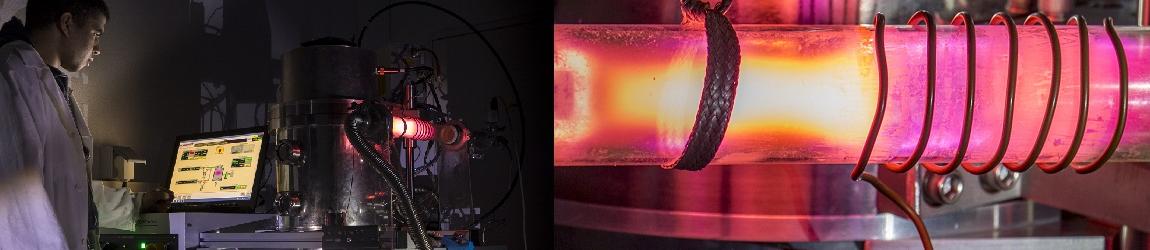
The X rays diffraction - XRD is a classical technique for identification and charaterization of the cristalline structure of materials, which started from the beginning of the XXth century and who has been continuously improved. In its ordinary version, used here, it allows to measure with precision the distances d(hkl) between different crystalline plans (hkl) of a crystal, from the measurement of the deviation angles 2 θ of an incident X ray beam. The setup has several components as a high voltage generator (60kV maximum), current (60 mA maximum), an X ray tube (copper, molybden, or cobalt…anticathode), a cooling water, a CCD sensor (before, Geiger counters or proportional were used), and an acquisition set aiming to drive the goniometer and record the diffractograms I = f( θ ).
The X ray tube and the CCD sensor arm rotate of an angle θ during the acquisition process.
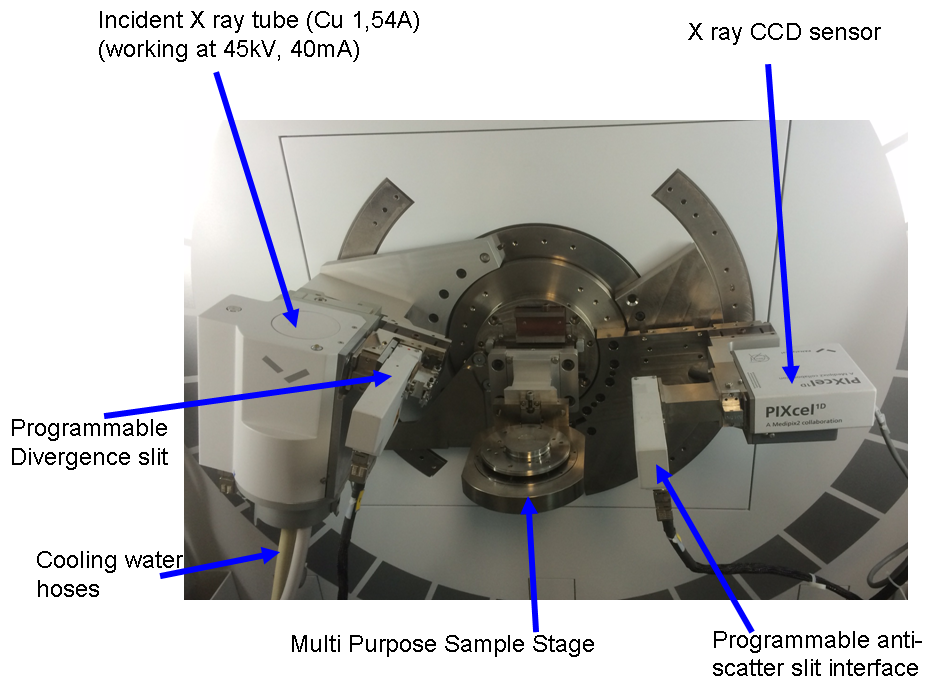
Figure 1 : view in details of the sample stage, the goniometric head, with the X Rays copper tube, the programmable slits and CCD sensor
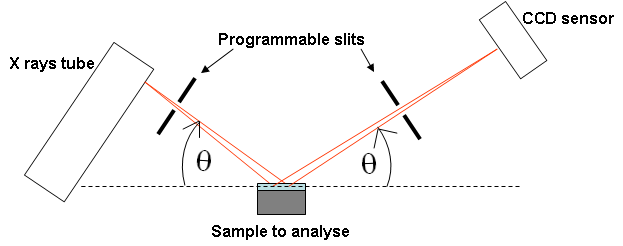
Figure 2 : Schematic of the X rays path in the goniometric setting
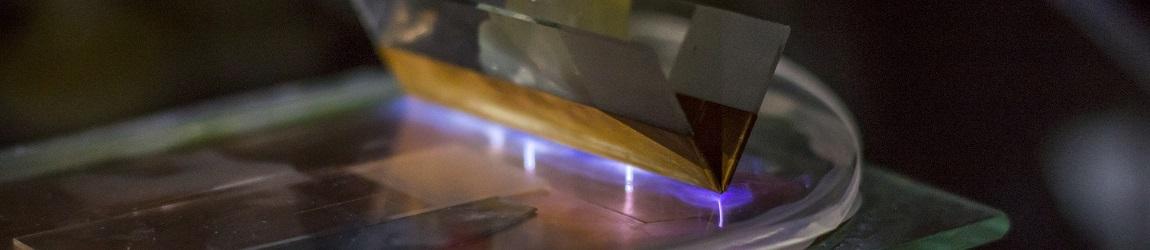
New techniques of grazing incidence and micro-diffraction are being developped on thin films obtained by electrochemical deposition.
Figure 3 : Grazing Incidence X Rays Diffraction - GIXRD diagram : the incident beam has a fixed very small angle regarding the sample surface
and the diffracted beam varies from 5° to 90° for analysing thin layers 10 nm to 500 nm by reducing the substrate response

Figure 4: The X Rays diffractometer Empyrean Panalytical
Identification and characterization of materials growth by an electrochemical process
- Cathodic electrolytic depositions of metals, alloys, composits…
- Anodic deposition of oxides
- Natural corrosion products or synthetized in the laboratory
- Precipitation by a pH shift from mineral compounds
- Materials used in electrochemical setups (accumulators, batteries, sensors...)
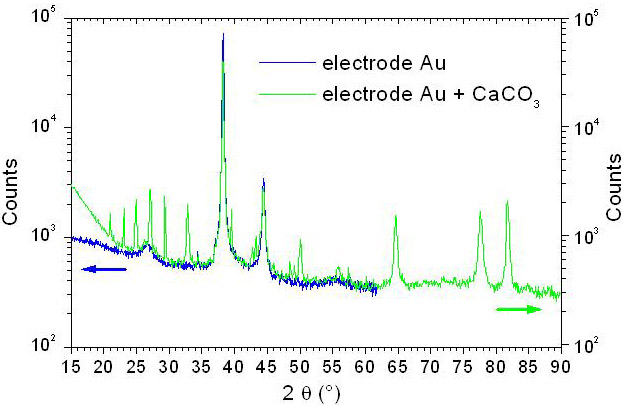
Figure 4: XRD spectra (Cu cathode 1.54 Å) of a calcite CaCO3 growth on gold substrate before and after deposition